
This year's return is 114%, Yongying Fund's Dan Lin: Investment in innovative drugs is no longer early-stage; we need to choose companies that can deliver the best results in the next one or two quarters

Shan Lin from Yongying Fund shared his views on innovative drug investments on September 18. He pointed out that current investment opportunities are mainly focused on the innovative drug sector with BD going overseas as the main line. Despite market fluctuations, the research and development of innovative drugs will continue, and policy disruptions are temporary, which may present a good buying opportunity. Shan Lin emphasized that investors should pay attention to companies that can deliver results in the next one or two quarters. The Yongying Pharmaceutical Innovation Select Mixed Fund has risen over 114% this year

Innovative drugs have recently experienced high-level fluctuations; what will the future trend be?
On the afternoon of September 18, Yongying Fund's Shan Lin shared his views on the investment, research, and overseas expansion of innovative drugs in a company program.
The investment representative summarized the key points as follows:
-
This wave is primarily an investment opportunity in innovative drugs with BD overseas expansion as the main line.
-
The research and development of innovative drugs in China will not stagnate due to a downturn in investment and financing or poor performance in the secondary market.
-
This is an opportunity driven by industrial trends, independent of anyone's will.
From a forward-looking perspective, all these policies' disturbances to the industry are temporary, and precisely during the disturbances and fluctuations, it may be a good buying point.
- At the current time point, we are no longer at the starting point; we indeed need to see more news about the industry and individual stocks that can be realized or fulfilled.
At the beginning of the year, when all assets were at relatively low levels, we might not be particularly concerned about details, as long as the general direction was correct, choosing some individual stock directions was acceptable.
But now, at this time point, everyone has risen. However, you cannot stop investing; you need to choose those companies with high confidence, realizable potential, and preferably those that can deliver results in the next one or two quarters.
- Companies that can better deliver results will lead in the second half of the competition.
Shan Lin, fund manager at Yongying Fund, has 4 years of experience in the securities industry. He previously served as a pharmaceutical researcher at Dongfang Securities Co., Ltd. and Changjiang Securities Co., Ltd., and joined Yongying Fund in 2023.
He manages a total fund asset scale of 3.143 billion yuan and currently manages two products: Yongying Pharmaceutical Health and Yongying Pharmaceutical Innovation Select.
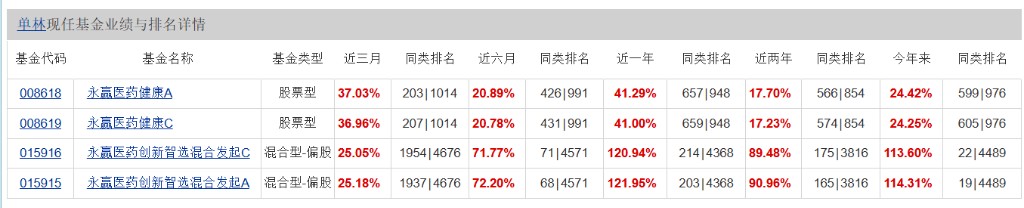
Yongying Pharmaceutical Innovation Select Mixed Fund focuses on the pharmaceutical industry, with a key layout in the innovative drug direction, and has performed outstandingly this year.
Among them, the A share of Yongying Pharmaceutical Innovation Select Mixed Fund has increased by over 114% this year, with a one-year return rate of 112%; in terms of short-term performance, it has risen by 72% in the past 6 months, and the cumulative return in the past 3 months is 25%.
On August 5, the representative shared Shan Lin's views on innovative drugs. Shan Lin believes that innovative drugs will still experience fluctuations, but the industrial trend remains unchanged, with three key directions. On August 28, in the disclosed mid-year report of the fund, Dan Lin once again pointed out that the upgrade of China's innovative drugs is not a "multiple-choice question," but a "mandatory question." Compared to the entire industry’s assets, innovative drugs still represent assets with relatively strong certainty, high confidence, and significant potential.
The industrial trend characterized by innovative drugs going global is expected to continue to unfold, with several heavyweight domestic innovative drugs likely to achieve Proof of Concept (POC) and demonstrate excellent product competitiveness on the world stage. Innovative drugs are expected to become one of the main investment themes in the entire market.
Dan Lin also stated that domestic innovative drugs are currently on the eve of releasing industrial dividends, and those that can truly cross the cycle will certainly be the enterprises that possess real hard technology capabilities and can continuously create clinical value.
Below are the highlights organized by the investment workbook representative (WeChat ID: touzizuoyeben), shared with everyone:
This wave is an investment opportunity in innovative drugs centered around BD
This round of innovative drug market actually started around the Spring Festival. I have mentioned in several previous live broadcasts that this year's pharmaceutical market, including the innovative drug sector, is unfolding against the backdrop of an overall oversold pharmaceutical sector over the past four years.
Looking back to the end of last year, the pharmaceutical sector was still shrouded in the gloom of a one-sided decline. In fact, from 2021 to 2024, the overall trend of the pharmaceutical sector has been relatively weak. The starting point of this wave of market is precisely due to its sufficiently low valuation level.
Investing in the secondary market is quite interesting; there is a saying that goes, "the longer the horizontal, the higher the vertical."
We see many Hong Kong stocks in the innovative drug sector, even 18A stocks, once fell below a PB of one, and even below the book net cash. This is a process of value restoration or value reassessment from a very low starting point.
Starting from the Spring Festival, the first to recover domestically was the AI-related sector, which is the technology sector. For example, after the domestic AI model DeepSeek achieved breakthroughs, the global perception of China's innovative assets has changed.
The first to be recognized and reassessed were the technology-related and AI-related sectors, and then pharmaceuticals were generalized into the technology sector, leading everyone to realize that AI in healthcare also deserves more attention.
However, in the earliest stage, innovative drugs did not stand at the center of the stage. So why did they gradually come to the forefront later? Because changes in the industry will ultimately be truly reflected in the secondary market.
Although the investment environment and experience in the pharmaceutical sector over the past four years have not been particularly good, the entire industry is undergoing very positive changes—China's innovative drug research and development will not stagnate due to a sluggish investment environment or poor performance in the secondary market.
On the contrary, it is precisely because the industry has been continuously advancing in the real world over the past four years, striving to create better molecules, that we have seen the prosperous situation of innovative drugs this year. There is a causal relationship here.
Of course, during the process, for example, at the end of the first quarter and the beginning of the second quarter, innovative drugs welcomed some major BD (business development)/external authorization projects, such as Pfizer introducing 3SBio's bispecific antibody assets, BMS introducing BioNTech's bispecific antibody assets, as well as various ADCs (antibody-drug conjugates), small molecule drugs, etc., which made the main line logic clearer and clearer This wave is primarily an investment opportunity in innovative drugs with BD going overseas as the main line.
Why is BD going overseas the main line? Because we know that while domestic medical insurance supports innovation, it indeed faces certain pressures.
The aging population is increasing, and the frequency of medical visits among the elderly is also rising. If the single transaction price gradually increases, the payment pressure on medical insurance will be greater.
Therefore, if we can truly "go out," earn foreign exchange, and earn US dollars, it fully demonstrates that our innovation is competitive.
At the same time, if we can perform well in the fully competitive market of the United States, earn back US dollars, and make a lot of money, then in the Chinese market, where the level of competition is far lower than that of the United States, we should be able to do even better—this is logically consistent.
Thus, this wave is an investment opportunity in innovative drugs centered around BD, and the above is a review of the trends in the first half of the year.
The volatility of innovative drugs is a good buying point
Of course, there have been many twists and turns in between, such as the tariff issues in April causing some disturbances, including the United States increasing tariffs, as well as various disruptions like controlling drug prices for raw materials and generic drugs.
But looking back, it is just as I have always firmly emphasized:
This is an opportunity driven by industrial trends, not subject to anyone's will.
From a future perspective, all these policies' disturbances to the industry are temporary, and precisely when disturbances occur and fluctuations arise may be relatively good buying points.
Because in investing, one cannot be too directional; it should be relatively contrarian. You cannot be more fearful than others when they are fearful, nor can you be more greedy than others when they are greedy; otherwise, it is difficult to achieve mid-term profit effects.
Investment in innovative drugs is no longer at the starting point; choose companies that can deliver results in the next one or two quarters
Not only in the pharmaceutical or innovative drug sector, but all types of assets in the entire market, including sectors and individual stocks, share commonalities; the underlying principles are the same.
The core statement is: there are no sectors that rise forever, nor individual stocks that fall continuously; there are fluctuations.
Innovative drugs have actually been very strong from the end of the first quarter of this year until July and August. However, from early or mid-August until now, they have been in a high-level fluctuation process.
There are various reasons for the fluctuations:
First, is there any asset in the entire market that can inspire more enthusiasm than it? For example, the recent CPO, as well as investment opportunities in gold brought about by expectations of interest rate cuts in the United States, are new hotspots or investment opportunities that can divert funds.
Second, from the current time point, we are no longer at the starting point; we indeed need to see more news about the industry and individual stocks that can be realized or delivered.
This is my gradually evolving viewpoint from the beginning of the year until now—at the beginning of the year, when all assets were at relatively low levels, we might not be particularly concerned about details, which is the so-called "the general is on the road, not chasing small rabbits"; as long as the general direction is correct, choosing some individual stock directions is acceptable.
But standing at this point in time, everyone has risen.
However, since everyone has risen, you cannot stop investing; you need to choose those companies with high confidence that can deliver results, preferably those that can deliver in the next one or two quarters Because only through realization, especially in the later stages of asset realization, can the true valuation of these companies be further reassessed or reshaped, and the future profitability can be reflected, including the confidence in DCF cash flow discounting will also be further enhanced.
This may be a very important point for these companies to showcase investment opportunities in the next step, which is "realization."
I actually mentioned this term during a live broadcast by Xinhua News Agency in May this year. Companies that can better realize will lead in the second half of the competition.
Outlook for Innovative Drug Market in Q4: Four Major Drivers Highlighted
There are many points to look forward to in Q4, let me summarize:
The first is academic conferences. Academic conferences are platforms for Chinese innovative companies to publicly showcase their innovative drug clinical data, allowing global companies and research institutions to see their achievements.
Why do this?
The first is to show off, to let everyone know that we are doing this, and that the data produced is very good and impressive.
If not disclosed, many potential overseas partners will find it difficult to understand your data, or even be unaware of the existence of your experiments.
Once disclosed, communication and contact can take place to explore the possibility of achieving overseas cooperation, which brings us back to our main line of business development (BD).
Therefore, without data disclosure, without data show out, there is no next step. This is the first point.
The second is medical insurance negotiations. Medical insurance negotiations take place every year in the second half of the year, and these negotiations are very important for the domestic innovative drug market.
The third is that the BD of Chinese innovative drugs will gradually be realized. In fact, today there is a company in the A-share market that has just reached a small nucleic acid drug BD transaction, and the stock price performance is quite good. It is essential to see tangible implementation.
The process we describe for the future of Chinese innovative drugs to innovate, iterate, and become the next generation of global blockbusters must be achieved step by step. I think this is very important in the second half of the year.
The fourth point is that as we move into Q4, we will see many companies gradually entering the profit cycle.
The reasons why people used to hesitate to invest in innovative drugs are that, first, they may not understand the targets well, and second, the companies are not profitable, making it difficult to anchor valuations—whether you say 15 times PE, 20 times PE, or 25 times PE, there must be profits there.
If there are no profits, how can the value of the drugs be measured? Therefore, early investments in such assets are mainly participated in by professional investors related to pharmaceuticals.
However, this year, next year, and the year after, once innovative drug companies become profitable, the investment framework will become particularly simple—just look at the financial statements.
As long as you have high growth, more investors will pay attention. Especially in the context of the overall macro environment not having particularly good backgrounds, scarce high-growth assets will definitely be sought after and will showcase their investment value.
Two Dimensions of Innovative Drug R&D, Four Fields
The R&D fields of Chinese innovative drug companies are very pragmatic. Pragmatism is reflected in two dimensions:
First, the patient population is large enough to solve real disease problems for patients. A larger patient population indicates greater future treatment areas, application space, and commercialization value for the drugs; Secondly, these areas are often key focus directions for many large foreign pharmaceutical companies.
Let me give a few examples: first is the oncology field. We know that large overseas MNCs (multinational pharmaceutical companies) have patented drugs represented by PD-1 monoclonal antibodies, such as K drug and O drug, which are about to face patent expiration.
So who can iterate? Many Chinese companies are doing this. Including the collaboration between Pfizer and 3SBio that I just mentioned, which is actually related to the layout of next-generation iterative drugs, such as PD-1/VEGF, PD-1/IL-15, PD-1/IL-2, etc. This is all very clear iterative drug logic.
Next is ADC (antibody-drug conjugates), which is a "molecular missile": the front is the antibody, the middle is the linker, and the back is the small molecule toxin.
It finds the tumor site like precision-guided munitions and releases small molecule toxins to kill. Chinese companies are very good at this and have achieved very large BD transactions, all of which are public information.
Next is the metabolic field, such as weight loss and chronic diseases like non-alcoholic fatty liver disease.
Why are these areas receiving attention? Because the population is indeed large enough. The best business model is actually a drug that most people can use; this must be the best drug. Looking back, apart from the small molecule oral drugs for COVID-19, the weight loss GLP-1 related drugs have the fastest growth in volume.
Furthermore, there are small nucleic acid drugs. I summarize the field of small nucleic acid therapy as "long-term management of chronic diseases." For example, some patients have hypertension and hyperlipidemia; in the past, they might have taken medication every day, but now there is a small nucleic acid drug that may only require administration once every six months or one injection to control the disease, greatly improving compliance.
So these areas, including the oncology, metabolic, small nucleic acid fields, and other chronic disease areas, Chinese companies are very well positioned and have strong targeting.
The targeting is reflected in two dimensions: first, targeting a large patient population, and second, targeting the patent cliff of large overseas pharmaceutical companies. These are the two dimensions.
Two Ways for Innovative Drugs to Go Global, Prefer the First "Borrowing Arrows with Straw Boats"
I divide the internationalization of innovative drugs into two dimensions:
The first dimension is BD, which is the so-called "borrowing arrows with straw boats" approach.
Most Chinese companies currently may not have enough financial, material, or human resources to invest in overseas market development and global clinical trials, so they need to "hug the thigh" and find those companies on the demand side that need to overcome the patent cliff—large overseas pharmaceutical companies like Merck, Bristol-Myers Squibb, Pfizer, AbbVie, etc.
By cooperating with them, Chinese products can undergo clinical development overseas and further commercialization.
However, the core premise of all BD agreements is drug efficacy. The development of Chinese drugs has changed significantly compared to five or ten years ago; the core path is that good efficacy is everything, and it must truly solve patient problems.
Large pharmaceutical companies spend a lot of money to develop your drugs for overseas markets; they cannot buy a placebo. Moreover, the drugs they buy are not starting from scratch; they need to iterate on products that have already generated tens of billions or even hundreds of billions of dollars in sales, and the efficacy must be better than the previous blockbuster products So the efficacy of the drug is the premise of everything.
Of course, there are many other issues involved here, such as the efficiency of clinical advancement, the solidity of clinical data, and the consistency of data between China and the United States, all of which are problems that need to be faced and solved during the BD process. This is the first dimension, the "borrowing arrows with a straw boat" BD dimension.
The second dimension is to rely on oneself for commercialization.
Relying on oneself for commercialization is really difficult, requiring a significant investment of funds, manpower, and material resources. Moreover, similarly, efficacy remains the cornerstone.
To sell on one's own, it is likely necessary to make the drug the best in its class worldwide. There are already such companies in China, and once successful, sales in the United States can lead to very non-linear growth—selling 100 million this year, possibly 500 million next year, and over a billion in the following years. But this places higher demands on both the company and the drug.
Therefore, regarding the current state of the Chinese pharmaceutical industry, the first approach (BD) may be more reasonable.
After all, conducting clinical trials in the United States can cost a patient up to four to five hundred thousand dollars, and a large clinical trial with several hundred participants can easily run into hundreds of millions of dollars.
Currently, not only in the pharmaceutical industry but across all sectors in China, making money is not that easy; everyone has to be very careful with their finances.
Thus, the first approach is more in line with the current situation of Chinese companies. Of course, if one chooses to go it alone, there are many challenges, including patent challenges from large overseas MNCs, doubts about efficacy, regulatory access, etc., all of which need to be overcome step by step.
Fortunately, these are gradually being realized. I believe we have already passed the stage from 0 to 1, and everyone should have confidence in the entire industry.
Source: Investment Workbook Pro Author: Wang Li
For more insights from industry leaders, please follow↓↓↓

Risk Warning and Disclaimer
The market has risks, and investment requires caution. This article does not constitute personal investment advice and does not take into account the specific investment goals, financial conditions, or needs of individual users. Users should consider whether any opinions, views, or conclusions in this article are suitable for their specific circumstances. Investing based on this is at one's own risk


